Celltrion remains hopeful for US approval of Herzuma, Truxima despite initial FDA turn-down
By Sohn Ji-youngPublished : April 6, 2018 - 15:38
South Korean biopharma company Celltrion expects to obtain US approval for its two biosimilar drugs referencing Roche’s Herceptin and Rituxan by the year’s end, despite the US Food and Drug Administration’s initial turn-down of the two drugs this week.
Celltrion said Thursday that the US FDA has issued Complete Response Letters for two of its proposed biosimilars -- Herzuma, referencing Herceptin and Truxima, referencing Rituxan -- and requested supplementary data on the products.
A CRL indicates that the FDA has finished its review of a new drug application and decided not to approve it in its present form.
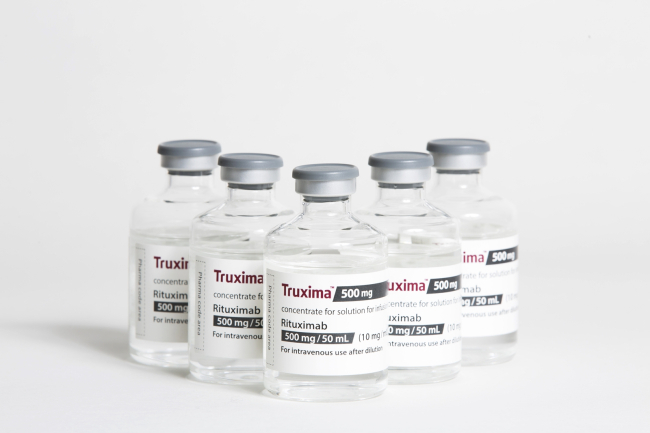
Responding to investor concerns, the Korean drugmaker said that it is working closely with the US regulatory agency to address issues that led to the CRLs, and expects to get the two drugs approved this year.
“Considering the US patent expiry dates for the two products, we have discerned that there won’t be significant delays in our originally planned product launch timetable,” Celltrion said in a statement.
The FDA’s move comes after a warning letter issued in January, raising issues over manufacturing processes such as deficient aseptic processing management and a lack of procedures to prevent microbiological contamination at its facilities in Songdo, Incheon, after the US regulator conducted an inspection from May 22 to June 2, 2017.
At the time, Celltrion said the warning letter did not affect its ability to manufacture and supply Inflectra -- its Remicade-referencing biosimilar -- citing confidence in the safety and efficacy of products manufactured at its Songdo production facilities.
Nonetheless, concerns had lingered that the FDA’s move would delay the approval of Herzuma and Truxima, both products awaiting US approval.
Meanwhile, Celltrion’s two biosimilars have been approved in the European Union. Truxima was approved in February 2017 while Herzuma was approved in February 2018. Both are currently sold across Europe via Celltrion’s marketing partners.
The Korean drugmaker has also been selling the Remicade-referencing biosimilar -- under the trade name Remsima and Inflectra -- in Europe since 2015 and in the US since October 2016.
By Sohn Ji-young (jys@heraldcorp.com)





![[KH Explains] No more 'Michael' at Kakao Games](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/28/20240428050183_0.jpg&u=20240428180321)











![[Herald Interview] Mistakes turn into blessings in street performance, director says](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/28/20240428050150_0.jpg&u=20240428174656)
