Articles by Lim Jeong-yeo

Lim Jeong-yeo
-
[KH Biz Forum] Watch your instincts to defend yourself: Kim Hoh
The worst path a company can go down in times of business crisis is to deny responsibility, attack victims, defend itself and delay a public apology, said Kim Hoh, founder and CEO of The LAB h, at The Korea Herald Biz Forum on Friday at the Shilla Seoul. The problem is that may just be what our brains are designed to do. Kim, citing socio-psychological research, illustrated that under stress, the brain’s emotional control system dominates over its reasonable decision-making capability,
Industry Oct. 11, 2019
![[KH Biz Forum] Watch your instincts to defend yourself: Kim Hoh](//res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2019/10/11/20191011000491_0.jpg&u=20191013154431)
-
Medytox appoints former acting US attorney for case against Daewoong
Medytox said Wednesday that it has appointed US attorney Joon H. Kim to represent it in a lawsuit against Daewoong Pharmaceutical in the US.Kim is a partner at Cleary Gottlieb, which focuses on white-collar criminal defense, internal corporate investigations, crisis management, commercial litigation and arbitration. Medytox emphasized Kim’s previous role as the acting US attorney for the Southern District of New York from March 2017 to January 2018, where he held one of the highest-l
Industry Oct. 9, 2019

-
NCSoft gives more info on Lineage2M launch
Lineage2M will release in Korea first, before going anywhere global, NCSoft said Tuesday. “We are not considering a simultaneous worldwide launch,” said Lee Sung-gu, a managing director at NCSoft, at a press event about the upcoming mobile massively multiplayer online role-playing game. This is the first new game NCSoft will release in two years. The exact launch date has yet to be announced, but character creation will open Oct. 15 to give select users a head start. Globally, in cou
Industry Oct. 8, 2019
-
Medytox says its BTX forms spores too
Medytox on Monday said its botulinum toxin (BTX) strain formed spores when tested according to the Daewoong Pharmaceutical way, in yet another turn amid the ongoing legal battle between the two firms.Medytox alleges Daewoong stole its BTX strain and trade-secret separation and purification method through a former Medytox employee who joined Daewoong.The firms have ongoing suits in Korea and the US. Daewoong’s BTX Nabota is making inroads to the US and Europe, under the names Jeuveau and Nu
Industry Oct. 7, 2019
-
Korean game companies offer unusual welfare programs
Once notorious for painstakingly long working hours and unfavorable conditions, game companies here are making a 180-degree-turn, offering workplace benefits that stretch beyond conventional areas.In-house day care for children, various classes to help employees develop their skills, gyms, libraries and lavish cafeteria meals are no longer unusual at these companies, according to industry sources.But Kakao Games and Pearl Abyss have other benefits that are more unusual. Kakao Games offers, among
Industry Oct. 6, 2019

-
Lancet publishes safety trial results for Yuhan's 3rd gen. cancer drug
The results of phase 1 and 2 clinical studies for Yuhan’s cancer drug lazertinib, suggesting that it has a “tolerable” safety profile and warrants further clinical investigation, were published in the prestigious journal the Lancet Oncology, the company said Friday.The Lancet, founded in 1823, is an international weekly medical journal.Lazertinib is an irreversible, third-generation, mutant-selective, EGFR tyrosine kinase inhibitor. Yuhan hopes it can provide another option for
Industry Oct. 4, 2019

-
More than 300 cases of Invossa side effects reported
More than 300 cases of Invossa side effects have been reported, the National Assembly’s Health and Welfare Committee said Friday. Invossa is the first-ever gene therapy drug from Korea that treats joint inflammation. Kolon Life Science acquired the sales approval of the drug in July 2017, only to have it revoked this May after the company admitted mislabeling the drug’s main ingredient.When applying for Invossa’s commercialization with the Ministry of Food and Drug Safety here,
Industry Oct. 4, 2019

-
Invossa aftereffects course over to other gene therapy companies
The Ministry of Food and Drug Safety inspected six gene therapy companies as a follow-up measure to the Invossa issue, a wire report said Thursday.Invossa, a gene therapy for joint inflammation treatment by Kolon Life Science, was stripped of its sales approval in May this year after the company belatedly reported a mislabeling of the key cell used in the drug. Invossa obtained the MFDS approval in July 2017 and started commercial sale in November of the same year.While attempting to make inroad
Industry Oct. 3, 2019

-
Daewoong’ BTX gains European approval
Daewoong Pharmaceutical’s botulinum toxin Nuceiva was greenlighted by the European Commission for commercialization, Daewoong said Wednesday. US’ Evolus, the exclusive partner of Daewoong, plans to roll out Nuceiva in Europe from 2020 as forehead wrinkle smoothening treatment. Nuceiva is known as Navota in Korea and Jeuveau in the US. Daewoong put emphasis on having made inroads to both US and Europe markets, which together account for approximately 70 percent of the world&rsqu
Industry Oct. 2, 2019

-
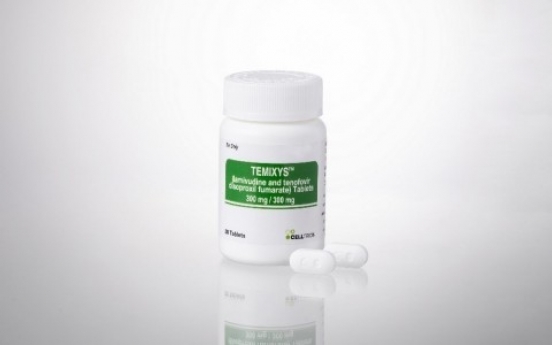
Celltrion to offer HIV treatment Temixys in US at lowest market rate
Celltrion will launch its human immunodeficiency virus treatment Temixys in the US at a significantly lower price, the company announced Tuesday. Temixys was approved in November 2018 by the US Food and Drug Administration. Celltrion said it expects the drug’s commercialization to start soon. The tablet is taken once daily and combines the two original chemical drugs Epivir and Viread. As original drugs, the cost for Epivir oral solution (10 mg/mL) is around $125 for a supply of 240 millil
Industry Oct. 1, 2019
-
Samsung BioLogics wins patent suit against Lonza
Samsung BioLogics said Tuesday it has won a lawsuit against Switzerland-based Lonza that is critical for manufacturing antibody agents in South Korea.Following the court ruling, domestic contract development pharmaceutical firms will be able to employ a more efficient method for cell culture production to boost their performance, a company official said.Cell culture is the process by which cells are grown under controlled conditions, generally outside their natural environment. The Korean firm h
Industry Oct. 1, 2019

-
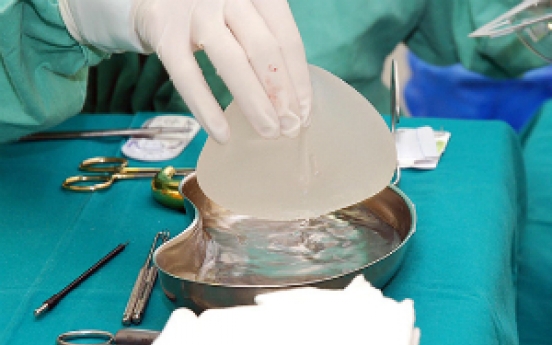
Allergan announcess compensation plan for breast implant patients
Allergan Korea on Monday rolled out compensation plans for patients treated with its breast implants, but fell short of expectations.The pharmaceutical firm’s rough-textured Natrelle Biocell Textured Breast Implants were red-flagged by the US Food and Drug Administration in July for having a sixfold risk of causing breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) -- a rare cancer of the immune system. Allergan has globally recalled all its products. The first Korean diag
Industry Sept. 30, 2019
-
Nexon to launch new mobile game V4 in Nov.
Korean game company Nexon said Saturday it will release a new mobile game titled V4 on Nov. 7, having already postponed a number of other games.Nexon had set out this year with ambitious plans to launch 10 new mobile games within the first half of 2019. However, the mobile version of The Kingdom of the Winds: Yeon, as well as SinoAlice, a Japanese game whose global distribution rights Nexon acquired, have both been postponed without being rescheduled. The Kingdom of the Winds: Yeon had a closed
Industry Sept. 29, 2019

-
Samsung strikes back at LG over legitimacy of QLED reference
Samsung Electronics on Sunday struck back at LG Electronics, saying its reference to quantum-dot light-emitting diodes for TV commercials had already been approved in major overseas markets.“It is regretful that the QLED reference is belatedly becoming an issue in Korea, when it had been cleared for use at major overseas markets,” said a Samsung representative. Samsung said the QLED label for its TVs had been approved by advertisement authorities in the US, the UK and Australia
Industry Sept. 29, 2019

-
Korea bans sales of ranitidine generics over carcinogen concern
South Korea’s Ministry of Food and Drug Safety tentatively put the brakes on the manufacturing, import and sale of 269 ranitidine-based drugs from 133 companies in the country, Thursday, upon alert from the US Food and Drug Administration that a carcinogenic chemical was found in the widely used medicine Zantac and its generic versions. Ranitidine is a drug that decreases stomach acid production. It is used to treat peptic ulcer disease and gastroesophageal reflux disease, or indigestion
Industry Sept. 26, 2019

Most Popular
-
1
Qoo10 liquidity crisis sparks massive complaints, fears of wider damage

-
2
Yoon urges municipalities to embrace foreigners

-
3
What is happening at Hybe?

-
4
S. Korea to consent to Japan's Sado mines gaining World Heritage status: official

-
5
Korea unveils tax reform bill to spur economy

-
6
Actor’s excessive airport security sparks probe into human rights violations

-
7
Actor Yoo Ah-in accused of sexual attack

-
8
Man who let his father die due to financial difficulties to be released on parole

-
9
S. Korea, China shifting from tensions to cooperation: Seoul

-
10
LG Electronics achieves record earnings in Q2



![[KH Biz Forum] Watch your instincts to defend yourself: Kim Hoh](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=649&simg=/content/image/2019/10/11/20191011000491_0.jpg&u=20191013154431)























