More than 300 cases of Invossa side effects reported
By Lim Jeong-yeoPublished : Oct. 4, 2019 - 15:42
More than 300 cases of Invossa side effects have been reported, the National Assembly’s Health and Welfare Committee said Friday.
Invossa is the first-ever gene therapy drug from Korea that treats joint inflammation. Kolon Life Science acquired the sales approval of the drug in July 2017, only to have it revoked this May after the company admitted mislabeling the drug’s main ingredient.
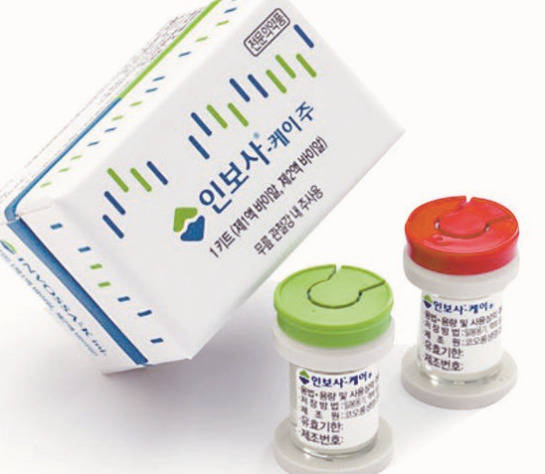
When applying for Invossa’s commercialization with the Ministry of Food and Drug Safety here, KLS had mislabeled Invossa’s cell line as cartilage-derived, while in fact it was a kidney-derived cell with tumorigenicity, a proliferation quality similar to that found in cancerous cells.
About 3,000 people have taken Invossa shots to help with knee trouble.
KLS has announced it would offer a 15-year follow-up package to patients who have taken the Invossa shot. The company maintains Invossa is safe after a radiation treatment in the drug development process.
However, according to the data submitted by the ministry to Health and Welfare Committee, there had been a total of 329 reported cases of side effects to the Korea Institute of Drug Safety and Risk Management between January 2014 and August 2018.
Included in the figures were eight cases of anomalies related to tumors and over 60 cases of no apparent efficacy.
The tumor-related cases had been voluntarily reported by medical professionals who had indicated that either the symptoms were irrelevant to Invossa or that they were unable to determine the cause. Due to this categorization, no epidemiological inspection has taken place to this day.
On Friday afternoon, local law firm OhKims filed a formal accusation of negligence against 11 Drug Ministry officials, including Minister Lee Eui-kyung.
Heads of Kolon Life Science and its US-based subsidiary Kolon TissueGene responsible for the research and development of Invossa are due in a hearing on Monday in the ongoing parliamentary audit.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)
Invossa is the first-ever gene therapy drug from Korea that treats joint inflammation. Kolon Life Science acquired the sales approval of the drug in July 2017, only to have it revoked this May after the company admitted mislabeling the drug’s main ingredient.

When applying for Invossa’s commercialization with the Ministry of Food and Drug Safety here, KLS had mislabeled Invossa’s cell line as cartilage-derived, while in fact it was a kidney-derived cell with tumorigenicity, a proliferation quality similar to that found in cancerous cells.
About 3,000 people have taken Invossa shots to help with knee trouble.
KLS has announced it would offer a 15-year follow-up package to patients who have taken the Invossa shot. The company maintains Invossa is safe after a radiation treatment in the drug development process.
However, according to the data submitted by the ministry to Health and Welfare Committee, there had been a total of 329 reported cases of side effects to the Korea Institute of Drug Safety and Risk Management between January 2014 and August 2018.
Included in the figures were eight cases of anomalies related to tumors and over 60 cases of no apparent efficacy.
The tumor-related cases had been voluntarily reported by medical professionals who had indicated that either the symptoms were irrelevant to Invossa or that they were unable to determine the cause. Due to this categorization, no epidemiological inspection has taken place to this day.
On Friday afternoon, local law firm OhKims filed a formal accusation of negligence against 11 Drug Ministry officials, including Minister Lee Eui-kyung.
Heads of Kolon Life Science and its US-based subsidiary Kolon TissueGene responsible for the research and development of Invossa are due in a hearing on Monday in the ongoing parliamentary audit.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)


















![[Today’s K-pop] Treasure to publish magazine for debut anniversary](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/07/26/20240726050551_0.jpg&u=)