Hanmi to present new nonalcoholic fatty drug treatment in Europe
By Lim Jeong-yeoPublished : Aug. 18, 2020 - 14:03
Hanmi Pharmaceutical said Tuesday that it will present its LAPSTriple Agonist as a treatment for nonalcoholic fatty liver disease at the International Liver Congress hosted by the European Association for the Study of the Liver.
Due to the global COVID-19 pandemic, this year’s EASL ILC is to be held online Aug. 27-29.
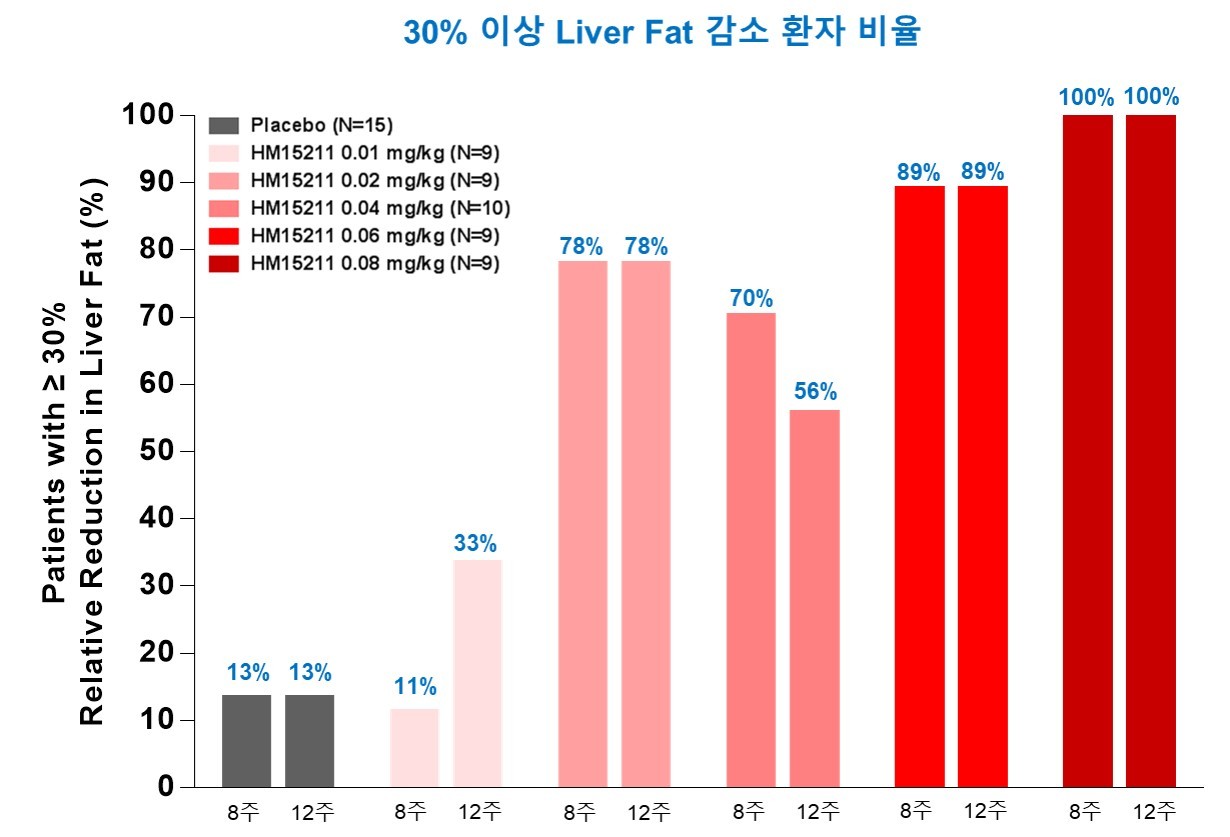
According to Hanmi, a 12-week repetitive administration of LAPSTriple Agonist in 66 obese patients with nonalcoholic fatty liver disease, also known as NASH, has shown that the patient cohort given the greatest dose had an average 81.2 percent reduction of fatty liver.
Across all cohorts, the program monitored a sweeping 50 percent reduction of fatty liver.
“This is the best known NASH treatment that is being developed in the world and has strong advantage against competing pipeline FXR agonist,” Hanmi said in a press release.
Hanmi’s discoveries will be introduced via two poster presentations and one oral session, to be delivered by Duke University’s Manal Abdelmalek as part of the late-breaking sessions.
Abdelmalek will present the results from preclinical and clinical phase 2a trials of Hanmi’s innovative platform that combines glucagon, GIP and GLP-1 in a single drug that can be swallowed.
Earlier in August, Hanmi licensed out the LAPSGLP/GCG dual agonist to American firm MSD, also as a nonalcoholic fatty liver treatment candidate.
Both the LAPSTriple Agonist and LAPSGLP/GCG have applied Hanmi’s independent platform technology Lapscovery.
Lapscovery refers to the Long Acting Protein and Peptide Discovery Platform Technology, which prolongs a biologic drug’s residence time in the human body. This cuts down on the number of times a patient must take the drug, potentially leading to optimized efficacy and decreased side effects of the treatment.
Hanmi has some 30 novel drug pipelines, of which 13 are being developed with Lapscovery technology.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)
Due to the global COVID-19 pandemic, this year’s EASL ILC is to be held online Aug. 27-29.
According to Hanmi, a 12-week repetitive administration of LAPSTriple Agonist in 66 obese patients with nonalcoholic fatty liver disease, also known as NASH, has shown that the patient cohort given the greatest dose had an average 81.2 percent reduction of fatty liver.
Across all cohorts, the program monitored a sweeping 50 percent reduction of fatty liver.
“This is the best known NASH treatment that is being developed in the world and has strong advantage against competing pipeline FXR agonist,” Hanmi said in a press release.
Hanmi’s discoveries will be introduced via two poster presentations and one oral session, to be delivered by Duke University’s Manal Abdelmalek as part of the late-breaking sessions.
Abdelmalek will present the results from preclinical and clinical phase 2a trials of Hanmi’s innovative platform that combines glucagon, GIP and GLP-1 in a single drug that can be swallowed.
Earlier in August, Hanmi licensed out the LAPSGLP/GCG dual agonist to American firm MSD, also as a nonalcoholic fatty liver treatment candidate.
Both the LAPSTriple Agonist and LAPSGLP/GCG have applied Hanmi’s independent platform technology Lapscovery.
Lapscovery refers to the Long Acting Protein and Peptide Discovery Platform Technology, which prolongs a biologic drug’s residence time in the human body. This cuts down on the number of times a patient must take the drug, potentially leading to optimized efficacy and decreased side effects of the treatment.
Hanmi has some 30 novel drug pipelines, of which 13 are being developed with Lapscovery technology.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)


















![[Today’s K-pop] Treasure to publish magazine for debut anniversary](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/07/26/20240726050551_0.jpg&u=)