[Health-tech Korea] Speclipse aims to increase early detection of skin cancer
By Sohn Ji-youngPublished : March 12, 2017 - 16:17
The Korea Herald is publishing a series of articles highlighting South Korea’s promising start-ups in the emerging sectors of digital health care and next-generation medical devices. This is the fourth installment. -- Ed.
While humans have yet to reach Mars, unmanned missions to the planet have already begun. Since 2012, NASA’s rover Curiosity has been studying the planet’s rocks and soils by using laser induced breakdown spectroscopy, or LIBS.
The technology works by shooting a high-energy laser pulse at a sample to form a plasma. By analyzing the light generated by the plasma, it immediately discerns the sample’s properties and composition.
While researching LIBS for similar robotic explorations, one mechanical engineer in South Korea began thinking — “could this same laser technology be applied to humans?”
That curiosity led Pyun Sung-hyun, founder and CEO of Speclipse, a Seoul-based startup, to develop a laser-based diagnostic system that uses LIBS to detect early symptoms of skin cancer, including melanoma.
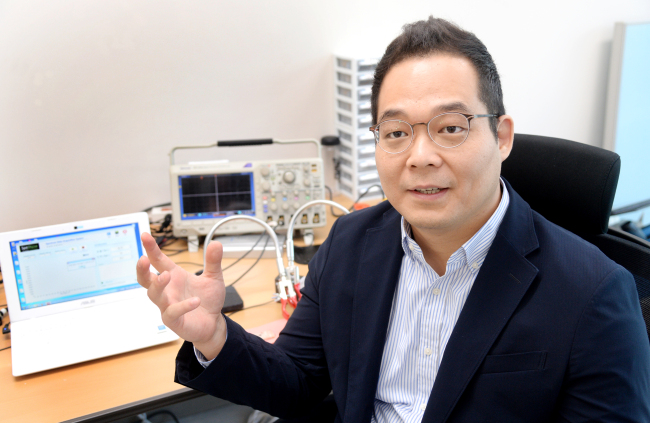
After earning a Ph. D in mechanical engineering from Stanford University, Pyun worked as consultant at BCG in Seoul before returning to research by joining the Korea Institute of Machinery & Materials as a plasma specialist in 2013.
It was there that Pyun partook in projects involving laser spectroscopy and its potential for use in clinical settings, including skin cancer diagnostics. To bring his idea to life, Pyun established Speclipse in November 2015.
The firm has built the Spectra-scope, which shoots a short laser pulse at a patient’s skin and immediately analyzes the chemical composition of the tissues to diagnose early-stage skin cancer.
The device’s module can be attached to the tip of conventional cosmetic lasers used by skin clinics and medical spas. The information collected by the module is sent to a small, box-shaped analytic device that uses computing algorithms to discern malignant skin marks with a 90 percent accuracy.
This is close to an accuracy of 95 percent currently offered by a biopsy —an “invasive” procedure which involves carving out more than 7 millimeters of the skin and costs around $1,000 without insurance, Pyun told The Korea Herald in an interview.
“We believe our exclusive technology will help doctors easily detect patients with skin cancer early on in places where the life-threatening disease is most prevalent, including Australia, the US and Canada,” the Speclipse CEO said.
According to Pyun, 2 in 3 Australians are diagnosed with skin cancer by the age of 70 in Australia, while 1 in 5 Americans develop skin cancer in their lifetime. In Canada, 1 in 5 are diagnosed with skin cancer at least once, he explained.
Despite the prevalence of skin cancer among populations in the West, the skin cancer diagnostics market remains largely undeveloped, with many patients left untreated until the cancer has significantly matured.
“Among US patients diagnosed with skin cancer, around 53 percent are diagnosed after the cancer has progressed beyond stages one, two and three,” he said.
Pyun, 36, hopes to change this by popularizing the Spectra-scope, which can be easily attached to existing laser equipment used by not only official dermatology hospitals but also general clinics and beauty spas that are more accessible to the public.
Speclipse holds high hopes for its portable device, which is more accurate and cheaper than existing diagnostic devices for skin cancer — produced by firms like MelaFind, Verisante Technology and VivoSight — that cost around $100,000 and offer lower diagnostic accuracy.
Investors have taken note of Speclipse’s potential as well. The medical device startup has attracted more than 3 billion won ($2.6 million) from local venture capital firms including Softbank Ventures Korea, InterVest and Mega Investment.
To bring the Spectra-scope to market, Speclipse has been working to conduct preliminary studies using a prototype of the device in partnership with local and overseas hospitals.
Once it debuts a finalized version of the device next month, the firm will start conducting clinical trials overseas. It will aim to first obtain regulatory approval in Europe and Australia by 2018 and later seek approval from the US Food and Drug Administration.
In the long run, Pyun believes its device can go beyond diagnosing skin cancer to detecting other skin conditions and cancers. By combining its core technology with machine learning, Speclipse hopes to move into other areas of medical diagnostics in the future as well.
By Sohn Ji-young (jys@heraldcorp.com)



![[AtoZ Korean Mind] Does your job define who you are? Should it?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/05/06/20240506050099_0.jpg&u=)













![[K-pop's dilemma] Is Hybe-Ador conflict a case of growing pains?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/05/07/20240507050746_0.jpg&u=)