Celltrion’s anti-COVID drug set for US, EU rollout: report
By Shim Woo-hyunPublished : Aug. 23, 2021 - 16:29
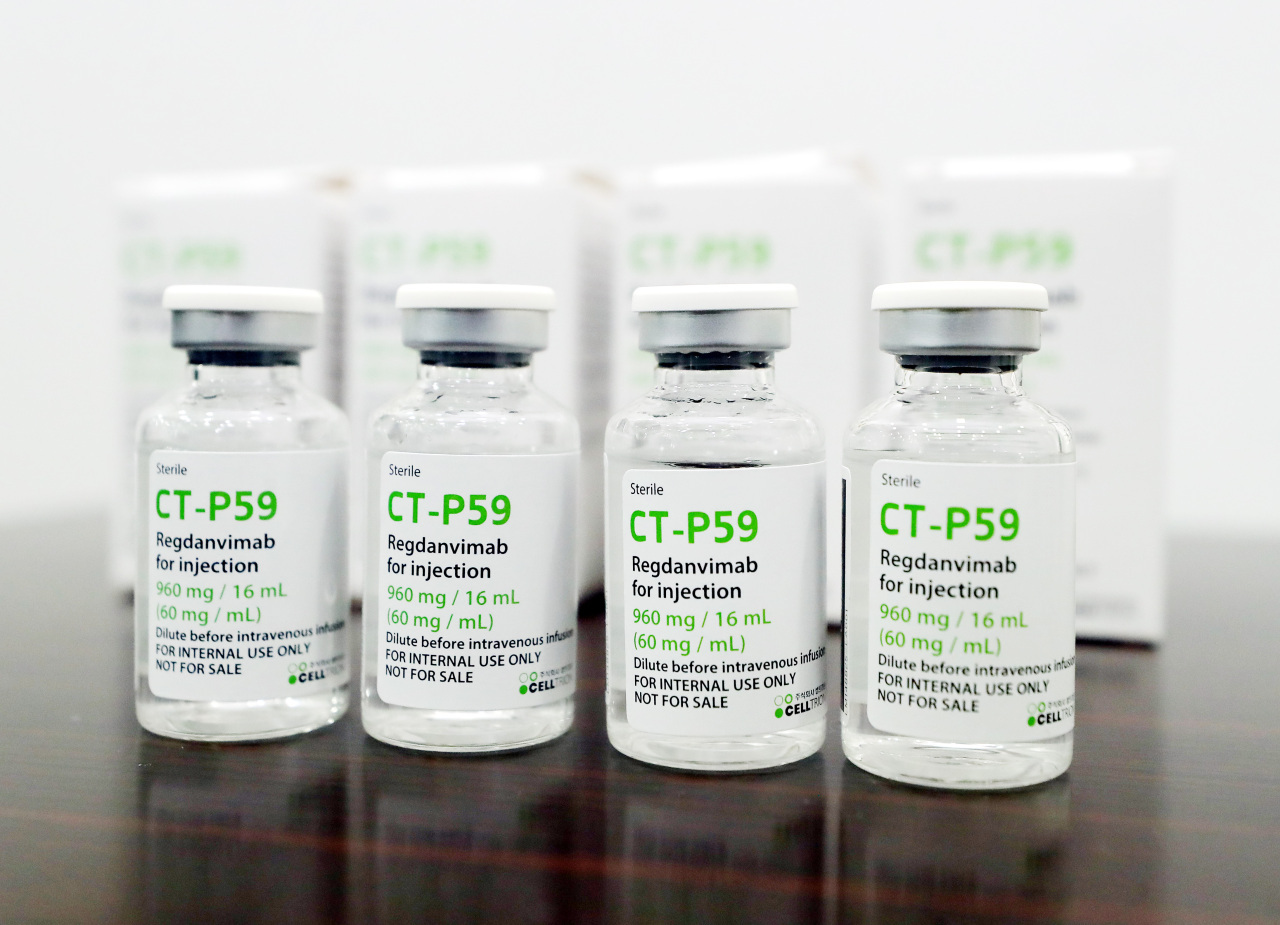
Celltrion’s COVID-19 antibody treatment, the world’s third therapy to be developed for the coronavirus, is likely to enter the US and European markets later this year, a local brokerage said in a report Monday.
According to Lee Dong-geon of Shinhan Investment, the South Korean pharmaceutical firm is likely to soon apply for the US Food and Drug Administration’s emergency use authorization for Regkirona, or CT-P59, based on the advisory meetings that the firm previously had with the US authority.
The market introduction of the anti-COVID-19 drug in the US could take place as early as the fourth quarter of this year, he predicted.
He added that in Europe, where the novel therapy is under a rolling review by the European Medicines Agency since February this year, Celltrion could receive regulatory approval before October.
A rollout of the drug in those major markets would improve the South Korean pharmaceutical firm’s bottom line in the coming months, the analyst added.
Rekirona, a monoclonal antibody treatment like ones developed by US companies Regeneron and Eli Lilly, was the first drug developed by a South Korean company against the coronavirus.
It received conditional approval from the South Korean drug safety agency in February and its first overseas approval from Indonesia in July. Recently, Brazil also gave the green light to the drug.
COVID-19 antibody drugs had largely gone unused in the initial months, but they are now back in demand as cases surge with the spread of the super-infectious delta variant across the world, Wall Street Journal reported recently.
Celltrion said it is currently in talks with several European countries to export Regkirona as soon as the EMA grants its final approval. The European authority in March has issued a positive scientific opinion for the drug.
Meanwhile, Shinhan Investment‘s report anticipated that sales of autoimmune biosimilar Remsima are expected to grow in the second half, boosting Celltrion’s earnings further.
Lee said Celltrion’s newly introduced adalimumab biosimilar Yuflyma could help the company to post improved earnings. During the second half, Yuflyma will be newly launched in some 10 more European countries, including Germany, France and Italy, Celltrion said in May.
Celltrion’s sales and operating profit could increase 27 percent and 35 percent, respectively, compared to the first half, according to Shinhan Investment’s report.






![[Weekender] How DDP emerged as an icon of Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/25/20240425050915_0.jpg&u=)



![[KH Explains] No more 'Michael' at Kakao Games](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/28/20240428050183_0.jpg&u=20240428180321)






![[Herald Interview] Mistakes turn into blessings in street performance, director says](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/28/20240428050150_0.jpg&u=20240428174656)
