[ANALYST REPORT] Hanmi Pharm: Beijing Hanmi’s R&D potential
By Korea HeraldPublished : June 20, 2016 - 18:40
Chinese government regulation to impact Chinese pharma market
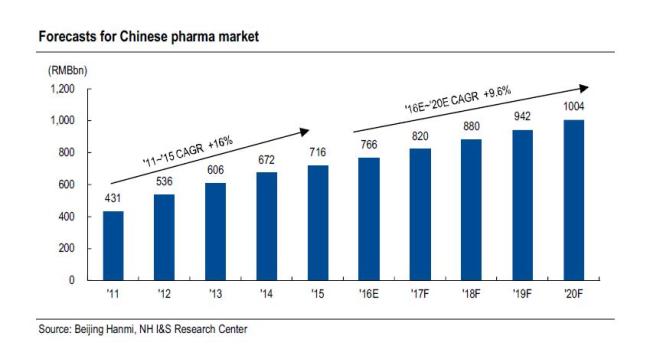
Over Jun 16~17, Hanmi Pharm’s Chinese subsidiary—Beijing Hanmi—invited analysts to a site visit for the first time in seven years. According to IMS data, the Chinese pharma market was worth RMB1.1tn in 2015 (grew at a 2011~2015 CAGR of 16.0%), and the market (according to IMS estimates) will grow at a 2016~2020 CAGR of 6.9%.
Growth factors for the Chinese pharma market: expanding health insurance coverage; growing private healthcare expenditure; and increasing prevalence of chronic diseases. Looking at negative factors: expanding health insurance premium deductions; a strengthened bidding system; price regulation; and national price negotiations for patented drugs.
The Chinese government has been strengthening regulation for the pharma sector, and new regulations include: simplifying the drug distribution process; strengthening the bidding system and cutting drug prices; retesting existing drugs’ bioequivalence, production equivalence, pharmacologic equivalence, and clinical equivalence; and moving towards a more rational medical system (ie, getting patients to visit small local clinics (rather than large hospitals) to treat minor ailments)
To overcome effects of regulation via R&D
Beijing Hanmi posted 2015 sales of RMB1,137.1mn (up 12.5% y-y), operating profit of RMB169.2mn (up 13.1% y-y), and net profit of RMB150.9mn (up 11.4% y-y). The firm’s R&D investments in 2015 came to RMB86.2mn (equal to 7.6% of its sales), while its sales rose at a 2006~2015 CAGR of 21.2%.
In 2015, after an investigation, the Chinese FDA (CFDA) pressured pharmas to cancel drug approval applications for drugs that lacked clinical trial evidence.
Almost all Chinese pharmas—including Beijing Hanmi—canceled their applications and restarted clinical trials (Beijing Hanmi plans to restart 50~70 clinical trials for the drugs that it withdrew and receive approval for these drugs in 2018). Elsewhere, new Chinese government price regulation is expected to result in Beijing Hanmi’s drug price falling 10% in 2016.
Beijing Hanmi has 10 R&D pipelines in the research and pre-clinical trial stages. The firm plans to develop—in the autoimmune disease and oncology fields—dual target antibody treatments and dual target synthetic drugs.
Chinese pharmas’ interest in new drug R&D pipelines is rising. Going forward, Beijing Hanmi plans to license out its products to global pharmas.
Source: NH Investment & Securities http://www.nhwm.com/
Over Jun 16~17, Hanmi Pharm’s Chinese subsidiary—Beijing Hanmi—invited analysts to a site visit for the first time in seven years. According to IMS data, the Chinese pharma market was worth RMB1.1tn in 2015 (grew at a 2011~2015 CAGR of 16.0%), and the market (according to IMS estimates) will grow at a 2016~2020 CAGR of 6.9%.
Growth factors for the Chinese pharma market: expanding health insurance coverage; growing private healthcare expenditure; and increasing prevalence of chronic diseases. Looking at negative factors: expanding health insurance premium deductions; a strengthened bidding system; price regulation; and national price negotiations for patented drugs.
The Chinese government has been strengthening regulation for the pharma sector, and new regulations include: simplifying the drug distribution process; strengthening the bidding system and cutting drug prices; retesting existing drugs’ bioequivalence, production equivalence, pharmacologic equivalence, and clinical equivalence; and moving towards a more rational medical system (ie, getting patients to visit small local clinics (rather than large hospitals) to treat minor ailments)

To overcome effects of regulation via R&D
Beijing Hanmi posted 2015 sales of RMB1,137.1mn (up 12.5% y-y), operating profit of RMB169.2mn (up 13.1% y-y), and net profit of RMB150.9mn (up 11.4% y-y). The firm’s R&D investments in 2015 came to RMB86.2mn (equal to 7.6% of its sales), while its sales rose at a 2006~2015 CAGR of 21.2%.
In 2015, after an investigation, the Chinese FDA (CFDA) pressured pharmas to cancel drug approval applications for drugs that lacked clinical trial evidence.
Almost all Chinese pharmas—including Beijing Hanmi—canceled their applications and restarted clinical trials (Beijing Hanmi plans to restart 50~70 clinical trials for the drugs that it withdrew and receive approval for these drugs in 2018). Elsewhere, new Chinese government price regulation is expected to result in Beijing Hanmi’s drug price falling 10% in 2016.
Beijing Hanmi has 10 R&D pipelines in the research and pre-clinical trial stages. The firm plans to develop—in the autoimmune disease and oncology fields—dual target antibody treatments and dual target synthetic drugs.
Chinese pharmas’ interest in new drug R&D pipelines is rising. Going forward, Beijing Hanmi plans to license out its products to global pharmas.
Source: NH Investment & Securities http://www.nhwm.com/
-
Articles by Korea Herald




















![[Today’s K-pop] Le Sserafim garners 100m views with ‘Eve’ music video](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/05/24/20240524050572_0.jpg&u=)