Celltrion’s Remsima SC authorized to treat rheumatoid arthritis in Korea
By Lim Jeong-yeoPublished : Feb. 26, 2020 - 11:26
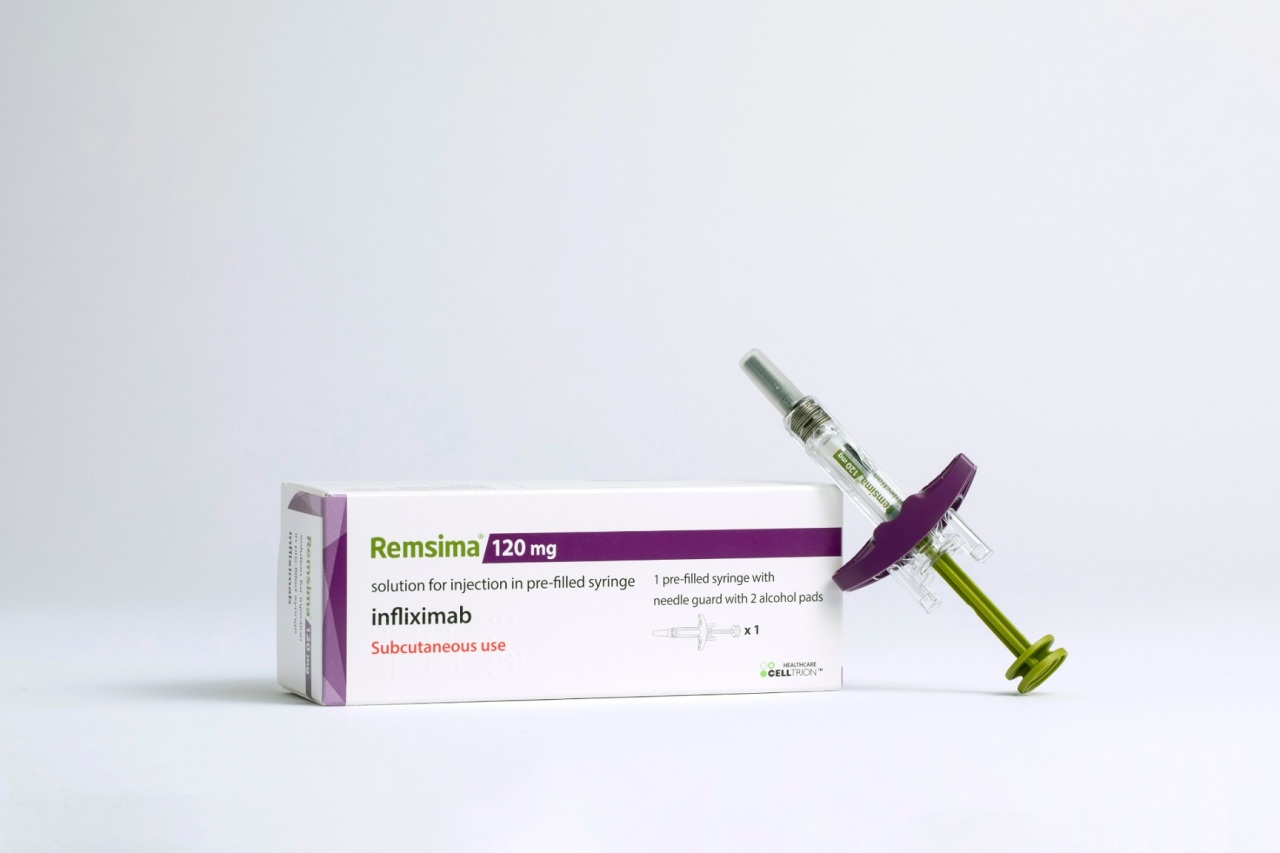
Celltrion’s autoimmune disease treatment Remsima SC was approved to treat rheumatoid arthritis in South Korea, the company announced Wednesday.
Remsima SC is a biobetter of Johnson & Johnson’s Remicade (infliximab), and is the world’s first subcutaenously injectable form of infliximab that comes in a prefilled syringe called an autoinjector.
However, while intravenous Remsima is authorized for treatment of psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, psoriasis, Crohn’s disease and rheumatoid arthritis, Remsima SC is authorized only for treatment of rheumatoid arthritis.
Celltrion is applying to expand the scope of Remsima SC’s treatments to cover inflammatory bowel disease, according to the firm. Sales of Remsima SC in Korea will be put on hold until Celltrion gets the IBD authorization from the Ministry of Food and Drug Safety.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)
Remsima SC is a biobetter of Johnson & Johnson’s Remicade (infliximab), and is the world’s first subcutaenously injectable form of infliximab that comes in a prefilled syringe called an autoinjector.
However, while intravenous Remsima is authorized for treatment of psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, psoriasis, Crohn’s disease and rheumatoid arthritis, Remsima SC is authorized only for treatment of rheumatoid arthritis.
Celltrion is applying to expand the scope of Remsima SC’s treatments to cover inflammatory bowel disease, according to the firm. Sales of Remsima SC in Korea will be put on hold until Celltrion gets the IBD authorization from the Ministry of Food and Drug Safety.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)


















![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)