[Kosdaq Star] Clinical-stage SillaJen rises to Kosdaq powerhouse
Among local Kosdaq-listed biotech powerhouses, a clinical-stage biotech firm SillaJen is noted for its signature oncolytic virus Pexa-Vec. Less than 11 months after going public, ...
By Son Ji-hyoungPublished : Oct. 15, 2017 - 16:10
This is the 43rd in a series of articles analyzing major companies traded on the tech-heavy Kosdaq market. -- Ed
While the top-tier stock market is riding on the global IT boom, the second-tier bourse is strongly dominated by the biopharma technology sector, with pharmaceutical players accounting for seven out of the top 10 shares in market cap.
Among the local pharmaceutical powerhouses, a clinical-stage biotech firm SillaJen is noted for its signature oncolytic virus Pexa-Vec.
Less than 11 months after going public in December 2016, SillaJen became the fourth-largest share on the second-tier Kosdaq market, by holding 3.07 trillion won ($2.72 billion) in market cap as of Friday, when SillaJen closed at 46,400 won. Foreign investors held 2 percent of SillaJen as of Wednesday.
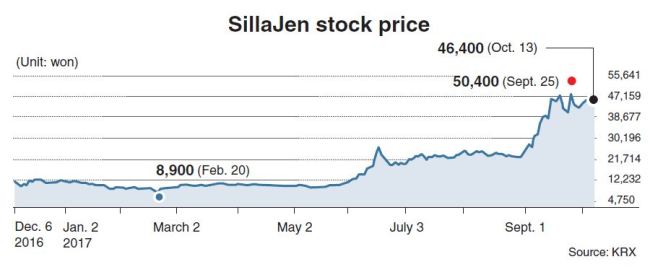
The American Society of Clinical Oncology’s annual meeting in early June began to shed light on the developer of the cancer cell-killing virus Pexa-Vec, also known as JX-594, prompting attention to the firm in the annual BIO International Convention held in San Diego, California, as well as a monthly stock surge in June by over 60 percent, from 12,500 won in May 31 to 20,050 in June 30. The price more than doubled in the next three months.
Such stellar performance on the Kosdaq market came on anticipation for the international Trial 3 for Pexa-Vec in advanced liver cancer, involving 600 patients.
If the Trial 3 for the therapy, a SillaJen-sponsored combination of Pexa-Vec and Bayer‘s kinase inhibitor drug Nexavar, completed in 2019 as planned, SillaJen would be able to commercialize its first drug by 2020, according to the firm. Nearly six out of 10 cancer therapies were known to have succeeded in Phase 3 clinical trials.
This came after the oncolytic immunotherapy gained approval for special protocol assessment by the US Food and Drug Administration in April 2015. The China FDA in July also approved of commencing Phase 3 trial.
Another combination, Pexa-Vec and REGN2810 by US biotech firm Regeneron going through a Trial 1 for kidney cancer therapy, is also garnering attention, with REGN2810, a cure for squamous cell carcinoma, designated as “breakthrough threapy” by the US FDA in September.
Starting Monday, SillaJen will recruit patients for Pexa-Vec-based therapy’s Trial I for colorectal cancer cure while partnering with US National Cancer Institute.
While the top-tier stock market is riding on the global IT boom, the second-tier bourse is strongly dominated by the biopharma technology sector, with pharmaceutical players accounting for seven out of the top 10 shares in market cap.
Among the local pharmaceutical powerhouses, a clinical-stage biotech firm SillaJen is noted for its signature oncolytic virus Pexa-Vec.
Less than 11 months after going public in December 2016, SillaJen became the fourth-largest share on the second-tier Kosdaq market, by holding 3.07 trillion won ($2.72 billion) in market cap as of Friday, when SillaJen closed at 46,400 won. Foreign investors held 2 percent of SillaJen as of Wednesday.

The American Society of Clinical Oncology’s annual meeting in early June began to shed light on the developer of the cancer cell-killing virus Pexa-Vec, also known as JX-594, prompting attention to the firm in the annual BIO International Convention held in San Diego, California, as well as a monthly stock surge in June by over 60 percent, from 12,500 won in May 31 to 20,050 in June 30. The price more than doubled in the next three months.
Such stellar performance on the Kosdaq market came on anticipation for the international Trial 3 for Pexa-Vec in advanced liver cancer, involving 600 patients.
If the Trial 3 for the therapy, a SillaJen-sponsored combination of Pexa-Vec and Bayer‘s kinase inhibitor drug Nexavar, completed in 2019 as planned, SillaJen would be able to commercialize its first drug by 2020, according to the firm. Nearly six out of 10 cancer therapies were known to have succeeded in Phase 3 clinical trials.
This came after the oncolytic immunotherapy gained approval for special protocol assessment by the US Food and Drug Administration in April 2015. The China FDA in July also approved of commencing Phase 3 trial.
Another combination, Pexa-Vec and REGN2810 by US biotech firm Regeneron going through a Trial 1 for kidney cancer therapy, is also garnering attention, with REGN2810, a cure for squamous cell carcinoma, designated as “breakthrough threapy” by the US FDA in September.
Starting Monday, SillaJen will recruit patients for Pexa-Vec-based therapy’s Trial I for colorectal cancer cure while partnering with US National Cancer Institute.

These are parts of SillaJen’s seven development pipelines using Pexa-Vec that is under clinical trials to fight various solid cancers, ranging from renal, colorectal, liver and lung cancer, by teaming up with global biotech firms such as Regeneron and France‘s Transgene.
SillaJen was one of the 33 biotech firms listed on the Kosdaq market that received special permission for initial public offering. Since 2005, the Korea Exchange has greenlighted IPO firms holding original technology.
SillaJen’s stock price surge originated from a global biotech boom, wrote William Ku, an analyst at NH Investment & Securities, citing Nasdaq Biotechnology index‘s gradual rise by nearly 25 percent this year.
Another analyst, Seo Mi-hwa of Yuanta Securities, wrote the market value of SillaJen’s liver cancer treatment is worth some 1 trillion won, while projecting SillaJen's liver cancer therapy market share to increase from 5 percent in the first year of commercialization to 30 percent 10 years later.
SillaJen was one of the 33 biotech firms listed on the Kosdaq market that received special permission for initial public offering. Since 2005, the Korea Exchange has greenlighted IPO firms holding original technology.
SillaJen’s stock price surge originated from a global biotech boom, wrote William Ku, an analyst at NH Investment & Securities, citing Nasdaq Biotechnology index‘s gradual rise by nearly 25 percent this year.
Another analyst, Seo Mi-hwa of Yuanta Securities, wrote the market value of SillaJen’s liver cancer treatment is worth some 1 trillion won, while projecting SillaJen's liver cancer therapy market share to increase from 5 percent in the first year of commercialization to 30 percent 10 years later.

Meanwhile, its earnings report have shown a stark contrast with the upbeat stock performance.
SillaJen has operated in the red since 2012. In 2016, SillaJen‘s operating loss came to 46.8 billion won, double that of 2015. As of the second quarter this year, the loss amounted to 27.2 billion won.
The operating loss stemmed from research and development costs, which stood at 18.9 billion won in the first half and 26.1 billion won in 2016, according to SillaJen. The entire cost of R&D is seen as expense, instead of assets, according to SillaJen‘s regulatory filing.
Dentist-turned-entrepreneur Moon Eun-sang has been serving as a chief executive officer of the firm since 2013. Moon is currently owning 7.95 percent of SillaJen.
By Son Ji-hyoung
(consnow@heraldcorp.com)
SillaJen has operated in the red since 2012. In 2016, SillaJen‘s operating loss came to 46.8 billion won, double that of 2015. As of the second quarter this year, the loss amounted to 27.2 billion won.
The operating loss stemmed from research and development costs, which stood at 18.9 billion won in the first half and 26.1 billion won in 2016, according to SillaJen. The entire cost of R&D is seen as expense, instead of assets, according to SillaJen‘s regulatory filing.
Dentist-turned-entrepreneur Moon Eun-sang has been serving as a chief executive officer of the firm since 2013. Moon is currently owning 7.95 percent of SillaJen.
By Son Ji-hyoung
(consnow@heraldcorp.com)



![[Herald Interview] 'Amid aging population, Korea to invite more young professionals from overseas'](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/24/20240424050844_0.jpg&u=20240424200058)

![[Pressure points] Leggings in public: Fashion statement or social faux pas?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/23/20240423050669_0.jpg&u=)













