Korea begins approval process for AstraZeneca vaccine
By Lim Jeong-yeoPublished : Jan. 4, 2021 - 15:20
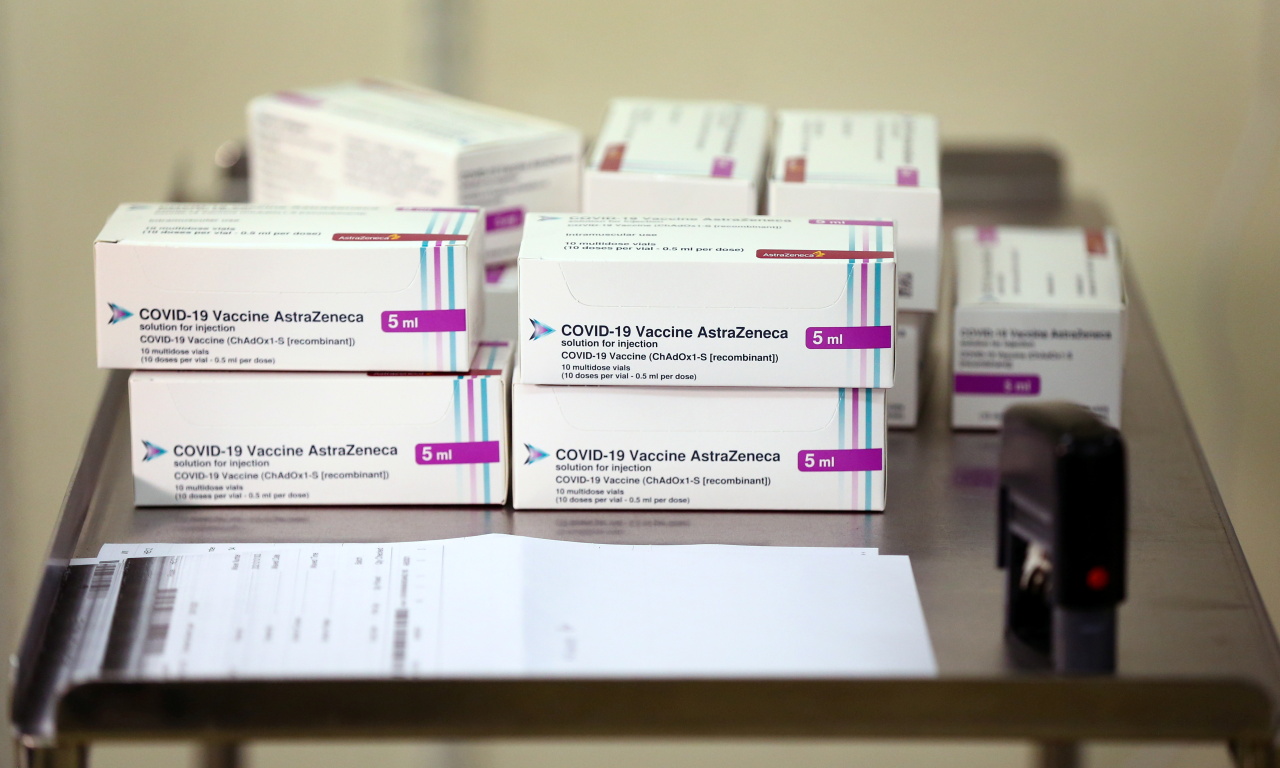
AstraZeneca’s COVID-19 vaccine (AZD1222) will be reviewed for potential approval in South Korea within the next 40 days, Korea’s Ministry of Food and Drug Safety announced Monday.
According to the Drug Ministry, AstraZeneca Korea has submitted applications for approval for its vaccine to be manufactured locally under a contract with SK Bioscience and for more of the vaccine to be shipped from manufacturers in Italy, the US and the UK.
AstraZeneca is required to submit further documents proving that the vaccine used in its clinical trials is of identical quality to that manufactured by SK Bioscience.
In the meantime, the Drug Ministry will undergo a rolling review of the applications to increase the time-efficiency of the approval process.
AstraZeneca’s vaccine is currently undergoing phase 3 clinical trials in some 10 countries including the UK, Brazil and the US. They are expected to be complete by September.
Once the trial is over the company is required to submit a 12-month follow-up detailing any reported side effects.
AstraZeneca’s is a viral vector vaccine, which stimulates the body to create neutralizing antibodies.
The vaccine is for those older than 18, and it needs to be given in two doses with a period of one to three months in between. It can be stored at temperatures between 2 and 8 degrees Celsius.
In the UK and Brazil, the test subjects included people over 65.
One subject experienced an unexpected spinal inflammation, and that stalled the trial in September. But after scientists declared that they had ruled out the vaccine as a cause, the trial resumed in the UK after a week and in the US after a month.
The UK granted emergency use approval for the AstraZeneca vaccine on Dec. 30, after the clinical trial targeting 11,636 test candidates proved its efficacy as a preventive.
The European Medicines Agency has been conducting a rolling review of the AstraZeneca vaccine since October.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)
According to the Drug Ministry, AstraZeneca Korea has submitted applications for approval for its vaccine to be manufactured locally under a contract with SK Bioscience and for more of the vaccine to be shipped from manufacturers in Italy, the US and the UK.
AstraZeneca is required to submit further documents proving that the vaccine used in its clinical trials is of identical quality to that manufactured by SK Bioscience.
In the meantime, the Drug Ministry will undergo a rolling review of the applications to increase the time-efficiency of the approval process.
AstraZeneca’s vaccine is currently undergoing phase 3 clinical trials in some 10 countries including the UK, Brazil and the US. They are expected to be complete by September.
Once the trial is over the company is required to submit a 12-month follow-up detailing any reported side effects.
AstraZeneca’s is a viral vector vaccine, which stimulates the body to create neutralizing antibodies.
The vaccine is for those older than 18, and it needs to be given in two doses with a period of one to three months in between. It can be stored at temperatures between 2 and 8 degrees Celsius.
In the UK and Brazil, the test subjects included people over 65.
One subject experienced an unexpected spinal inflammation, and that stalled the trial in September. But after scientists declared that they had ruled out the vaccine as a cause, the trial resumed in the UK after a week and in the US after a month.
The UK granted emergency use approval for the AstraZeneca vaccine on Dec. 30, after the clinical trial targeting 11,636 test candidates proved its efficacy as a preventive.
The European Medicines Agency has been conducting a rolling review of the AstraZeneca vaccine since October.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)

















![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)