Samsung Bioepis gains approval to sell bigger-size Ontruzant in Europe
By Lim Jeong-yeoPublished : March 5, 2019 - 12:12
Samsung Bioepis has gained approval to sell bigger-size Ontruzant in Europe, the company said Tuesday.
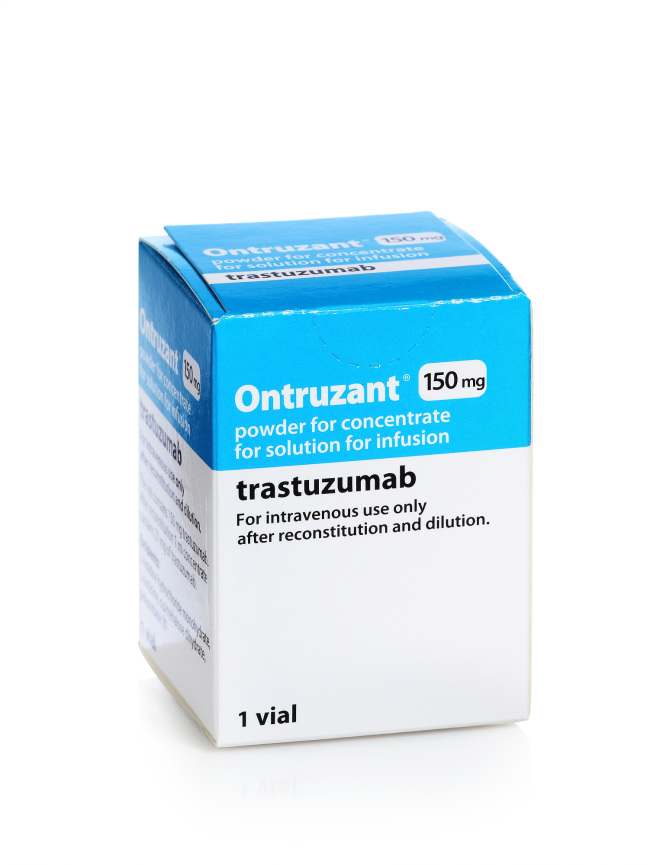
Ontruzant is a biosimilar for breast cancer treatment referencing Swiss firm Roche’s blockbuster drug Herceptin.
Available as a powder used to make up a solution for adding to an infusion into a vein, the 150 milligram version hit shelves in Europe in March 2018, after gaining European Medicine Agency’s approval in November 2017. The firm can now sell 420mg versions of the biosimilar.
Ontruzant is a biosimilar for breast cancer treatment referencing Swiss firm Roche’s blockbuster drug Herceptin.
Available as a powder used to make up a solution for adding to an infusion into a vein, the 150 milligram version hit shelves in Europe in March 2018, after gaining European Medicine Agency’s approval in November 2017. The firm can now sell 420mg versions of the biosimilar.

“In Europe, there is demand for both the 150mg and 420mg of Ontruzant,” said a Samsung Bioepis representative. With the latest EMA approval, Samsung Bioepis said it hopes to provide more diverse and effective treatment options for patients.
The biosimilar is currently being sold in 10 European nations. In September 2018, Samsung Bioepis won a 12.7 million euros deal to provide Ontruzant to four French hospitals.
Other than Ontruzant, Korean biopharma firm Celltrion’s Herzuma, also referencing the original Roche drug, was launched in Europe in the second quarter of 2018, taking up 6 percent market share in three months since release.












![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)




![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240418181020)

![[Today’s K-pop] Zico drops snippet of collaboration with Jennie](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/18/20240418050702_0.jpg&u=)