FDA denies Medytox’s petition against Daewoong’s botulinum strain
Medytox maintains claim against Daewoong, says will continue domestic, ITC suits
By Lim Jeong-yeoPublished : Feb. 20, 2019 - 16:40
The US Food and Drug Administration has denied a petition filed by South Korea’s Medytox demanding disclosure of the source of Daewoong Pharmaceutical’s botulinum toxin (BTX) strain used in Nabota, Daewoong said Wednesday, adding it will enter the US market without a change in plans.
Nabota, or Jeuveau outside Korea, is an affordable alternative to Allergan’s Botox, and the first Korea-made frown-line treatment to enter the US.
Nabota, or Jeuveau outside Korea, is an affordable alternative to Allergan’s Botox, and the first Korea-made frown-line treatment to enter the US.
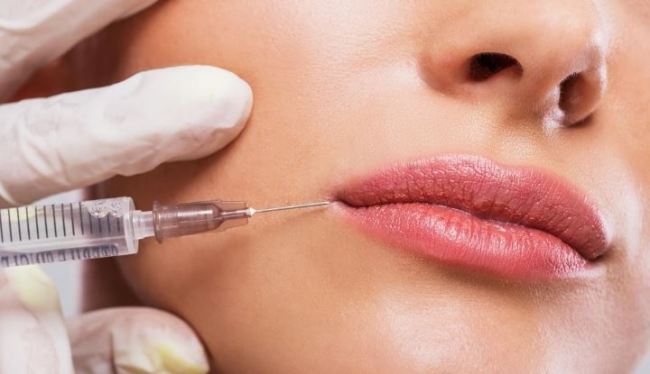
In response, Medytox said it will continue to push forward with lawsuits filed in Korea and at the International Trade Centre in Switzerland. It has been seeking to halt approval for Nabota, demanding that Daewoong identify and disclose the source of Nabota’s BTX, which Medytox alleges was stolen. If Medytox wins these suits, it can press for an injunction to halt production of Daewoong products.
The FDA on Feb. 1 denied Medytox’s petition, citing Daewoong’s US partner Evolus’ “trade secret or confidential commercial or financial information.”
Click to read full response from FDA.
The FDA added that its Application Integrity Policy is only invoked when there is a “pattern or practice of wrongful conduct that raises a significant question about the reliability of the data” supplied by firms.
According to the FDA, “The Agency reviewed ... and concluded the data provided by Evolus was sufficient to support approval” and that despite allegations raised in the petition, no wrongful acts were identified that would warrant invoking the policy. Medytox’s petition was filed on Dec. 5, 2017.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)








![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)





![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)