Kolon-owned TissueGene gets green light for Kosdaq listing
By Sohn Ji-youngPublished : Sept. 1, 2017 - 15:56
TissueGene, the US-based biotechnology subsidiary of South Korea’s Kolon Group, has received the green light from the local bourse regulator to begin its public listing procedures on the Kosdaq.
The Korea Exchange announced Thursday it has approved TissueGene’s preliminary application for listing on the Kosdaq, kick-starting the firm’s initial public offering process.
The news prompted shares of Kospi-traded Kolon to surge by 5.27 percent from the previous day to close at 69,900 won ($62.30) on Friday.
TissueGene is offering 1.5 million new shares for sale at an indicative price range of 16,000 won to 27,000 won, aiming to raise between 120 billion won to 202.5 billion won.
Local analysts have forecast TissueGene’s market capitalization to reach as high as 2 trillion won upon after its stock market debut. However, this figure may be dimmed depending on the market’s evaluation of the company’s main drug and its commercial value.
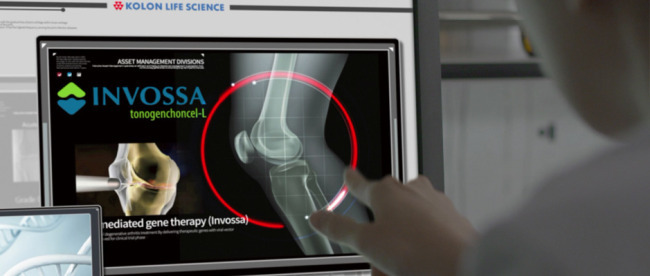
TissueGene, which is 31 percent owned by Kolon, developed Invossa, Korea’s first cell-mediated therapy for degenerative osteoarthritis, which was approved by the local Ministry of Drug and Food Safety in July.
Invossa is a first-in-class injectable cell gene therapy that treats osteoarthritis, a degenerative joint disease in which the cartilage that cushions the ends of bones between the joints breaks down.
Despite the approval, expectations for the drug’s future prospects were dampened after the drug was approved only for pain relief and function improvement in Korea.
The cell gene drug had been widely expected to offer breakthrough improvements in joint structure by regenerating damaged cartilage. It was a core therapeutic benefit that Kolon had stressed for Invossa as well.
The Kolon-owned drug developer has stated that its arthritis treatment will demonstrate its efficacy in joint improvement, including joint regeneration, through future phase 3 clinical trials involving 1,020 patients in the US.
By Sohn Ji-young (jys@heraldcorp.com)
The Korea Exchange announced Thursday it has approved TissueGene’s preliminary application for listing on the Kosdaq, kick-starting the firm’s initial public offering process.
The news prompted shares of Kospi-traded Kolon to surge by 5.27 percent from the previous day to close at 69,900 won ($62.30) on Friday.

TissueGene is offering 1.5 million new shares for sale at an indicative price range of 16,000 won to 27,000 won, aiming to raise between 120 billion won to 202.5 billion won.
Local analysts have forecast TissueGene’s market capitalization to reach as high as 2 trillion won upon after its stock market debut. However, this figure may be dimmed depending on the market’s evaluation of the company’s main drug and its commercial value.
TissueGene, which is 31 percent owned by Kolon, developed Invossa, Korea’s first cell-mediated therapy for degenerative osteoarthritis, which was approved by the local Ministry of Drug and Food Safety in July.
Invossa is a first-in-class injectable cell gene therapy that treats osteoarthritis, a degenerative joint disease in which the cartilage that cushions the ends of bones between the joints breaks down.
Despite the approval, expectations for the drug’s future prospects were dampened after the drug was approved only for pain relief and function improvement in Korea.
The cell gene drug had been widely expected to offer breakthrough improvements in joint structure by regenerating damaged cartilage. It was a core therapeutic benefit that Kolon had stressed for Invossa as well.
The Kolon-owned drug developer has stated that its arthritis treatment will demonstrate its efficacy in joint improvement, including joint regeneration, through future phase 3 clinical trials involving 1,020 patients in the US.
By Sohn Ji-young (jys@heraldcorp.com)










![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)
![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)





![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

