[DECODED: CELLTRION] Celltrion to face stiff competition in biosimilars business
‘First mover’ advantage may fade sooner than expected, experts say
By KH디지털2Published : Aug. 31, 2016 - 17:46
[THE INVESTOR] South Korea’s Celltrion is one of the hottest companies to watch in the biopharmaceuticals world, with soaring share prices and rising expectations, but it may soon find itself being chased by competitors.
Founded in 2002, the Korean drugmaker is considered a pioneer in the world’s emerging market for biosimilar drugs -- cheaper, near-replicas of expensive biologic drugs that have lost patent protection.
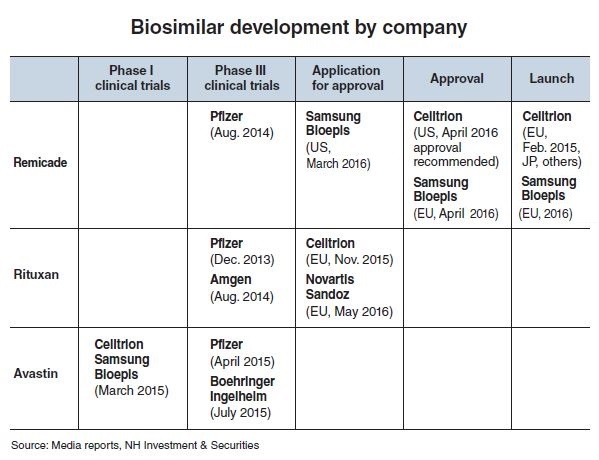
Having jumped into the field earlier than others, Celltrion introduced the world’s first biosimilar referencing Remicade in Europe in February last year. Celltrion’s Remsima, also known as Inflectra, is a cheaper alternative to Remicade, Johnson & Johnson’s blockbuster drug used to treat Crohn’s disease, ulcerative colitis, rheumatoid arthritis and other conditions.
Today, Celltrion is on track to commercialize Inflectra in the US with local sales partner Pfizer in as early as October, having won a key patent dispute against J&J in a US court last week, after scoring regulatory approval from the US Food and Drug Administration in April.
It is also preparing in the near future to launch two additional biosimilars -- one based on Rituxan and another on Herceptin -- that could become the first of their kind to launch worldwide.
Analysts say Celltrion’s core competitiveness comes from being a “first mover” -- being the first to launch a biosimilar version of a given biologic drug to singlehandedly siphon away the original drug’s sales in Europe and the US, the top two biologic markets in the world.
Related stories:
Founder’s era continues at Celltrion
Enigma of Seo Jung-jin
However, it may soon be facing stiff competition from other companies moving quickly to launch alternative biosimilars that overlap with Celltrion’s product lineup for those two key overseas markets.
Competition heats up for Remicade alternatives
Though Celltrion got a head start with Remsima in Europe, it is no longer the only company selling a Remicade biosimilar in the region.
In April this year, Samsung Bioepis, the biosimilars developing unit of Korean electronics giant Samsung Group, scored approval from the European Medicines Agency to commercialize its own Remicade biosimilar -- Flixabi, also known as Renflexis.
Samsung Bioepis’ European sales and marketing partner Biogen is swiftly working to introduce Flixabi across Europe, going in direct competition against Celltrion’s Remsima as well as J&J’s original drug. As of the second quarter of 2016, Celltrion’s Remsima had taken away some 40 percent of the original’s sales in Europe as the only Remicade biosimilar available in the region, the company said. And it claims it can drive this number up to 50 percent by the year’s end.
“As a ‘first mover’ product, Remsima has been accumulating prescription data over several years, boosting its credentials among both doctors and patients,” Celltrion said in a statement. “Given this, Remsima could take away some 50 percent of the original drug’s sales this year.”
However, the market entry of Samsung’s Flixabi could dim Remisma’s sales outlook in Europe, as it presents widened options to doctors treating patients diagnosed with conditions which require Remicade.
So far, Flixabi has been commercialized in Germany, one of the biggest markets for biologics within Europe, with plans for further launches across Europe, Biogen told The Korea Herald on Wednesday.
Moreover, analysts say Remsima’s performance is impressive, yet limited in scope. Celltrion’s replicated drug has had little impact on the sales of blockbusters drugs Enbrel and Humira, as was hoped, Samsung Securities analyst Kim Seung-woo pointed out in a February report.
Kim had forecast that Enbrel and Humira could be prescribed interchangeably with Remicade to treat autoimmune diseases like rheumatoid arthritis and ankylosing spondylitis, suggesting expanded market reach for Celltrion’s Remicade biosimilar, Kim said.
However, doctors in Europe appear to be cautious in prescribing Remsima in place of other anti-TNF biologic drugs (including Enbrel and Humira), given biosimilars are still new to the market, the analyst said.
Furthermore, biosimilar replications of the two drugs, produced by other global biopharma developers, have already entered, or are close to entering markets in Europe.
This year, the EMA approved two Enbrel biosimilars, each produced by Samsung Bioepis and Novartis-owned Sandoz. The former began selling its Enbrel replica -- Benepali -- in Europe from February, while the latter is making due preparations.
As for Humira, US pharmaceuticals giant Amgen and Samsung Bioepis are both awaiting the EMA’s permission to launch their Humira biosimilars.
“With the entry of Samsung’s Enbrel biosimilar this year and the debut of Humira biosimilars next year, Remsima’s sales will be further limited solely to the Remicade market,” Kim said.
Meanwhile in the US, Celltrion’s Remsima is poised to become the first Remicade biosimilar to be sold in the country, for the time being.
Having won a key patent dispute against Remicade’s original maker Janssen, a subsidiary of J&J, Celltrion and its sales partner Pfizer say they are on track in launching Remsima, to be sold under the name Inflectra, in the US by October.
The Korean drugmaker said it expects Inflectra to channel in some 2 trillion won ($1.79 billion) in annual profits in the US, taking away roughly 30 percent of Remicade’s US sales estimated at 5.4 trillion won.
Inflectra is expected to be sold at a price that is 15 to 20 percent cheaper than the original Remicade, HMC Investment Securities analyst Kang Yang-koo said in a June report.
Despite its early lead, Celltrion is now being chased by Samsung Bioepis, which applied for the US FDA’s regulatory approval of its Remicade biosimilar in March of this year.
Kang forecasts the Samsung unit will take around two years -- one year to gain regulatory approval and another year for commercialization preparations -- to officially debut its Remicade biosimilar in the US market, as was the case with Celltrion.
Race for additional biosimilars
Looking ahead, Celltrion is poised to become the first company to debut two additional biosimilars by as early as next year -- one referencing Roche’s lymphatic cancer treatment Rituxan and another based on Roche’s breast cancer drug Herceptin.
Despite its initial lead, the Korean drug maker may soon lose its “first mover” status as its two biosimilars again face quick-moving competition from the world’s biggest pharmaceuticals giants such as Novartis and Amgen.
Celltrion submitted its Rituxan biosimilar -- Truxima -- to the EMA in November 2015 and expects the European regulatory body to approve the drug by the year’s end. The firm plans to submit Truxima to the US FDA by the first quarter of 2017 as well.
Trailing behind by about six months in the segment is Novartis-owned Sandoz, which submitted its own Rituxan biosimilar to the EMA for approval in May of this year, and is expected to do the same for the US FDA in the near future.
Global pharmaceuticals giants Pfizer and Amgen are also wrapping up third-phase clinical trials of their own Rituxan biosimilars, with plans to commercialize their drugs in both Europe and the US in the coming future.
Meanwhile, no company has yet to seek regulatory approval of a Herceptin biosimilar in both Europe and the US, though Celltrion may become the first to do so.
The Korean biopharma developer said it plans to submit its Herceptin biosimilar -- Herzuma -- to the EMA before September. Celltrion began Herzuma’s third-phase clinical trials back in December 2011.
Set to quickly follow in the EU approvals process is Amgen, which began its third-phase clinical trials months later in July 2012. Pfizer and Samsung Bioepis are also preparing to launch their own Herceptin biosimilars, though their launches are expected at a slower pace.
By Sohn Ji-young/The Korea Herald (jys@heraldcorp.com)
Founded in 2002, the Korean drugmaker is considered a pioneer in the world’s emerging market for biosimilar drugs -- cheaper, near-replicas of expensive biologic drugs that have lost patent protection.
Having jumped into the field earlier than others, Celltrion introduced the world’s first biosimilar referencing Remicade in Europe in February last year. Celltrion’s Remsima, also known as Inflectra, is a cheaper alternative to Remicade, Johnson & Johnson’s blockbuster drug used to treat Crohn’s disease, ulcerative colitis, rheumatoid arthritis and other conditions.

Today, Celltrion is on track to commercialize Inflectra in the US with local sales partner Pfizer in as early as October, having won a key patent dispute against J&J in a US court last week, after scoring regulatory approval from the US Food and Drug Administration in April.
It is also preparing in the near future to launch two additional biosimilars -- one based on Rituxan and another on Herceptin -- that could become the first of their kind to launch worldwide.
Analysts say Celltrion’s core competitiveness comes from being a “first mover” -- being the first to launch a biosimilar version of a given biologic drug to singlehandedly siphon away the original drug’s sales in Europe and the US, the top two biologic markets in the world.
Related stories:
Founder’s era continues at Celltrion
Enigma of Seo Jung-jin
However, it may soon be facing stiff competition from other companies moving quickly to launch alternative biosimilars that overlap with Celltrion’s product lineup for those two key overseas markets.
Competition heats up for Remicade alternatives
Though Celltrion got a head start with Remsima in Europe, it is no longer the only company selling a Remicade biosimilar in the region.
In April this year, Samsung Bioepis, the biosimilars developing unit of Korean electronics giant Samsung Group, scored approval from the European Medicines Agency to commercialize its own Remicade biosimilar -- Flixabi, also known as Renflexis.
Samsung Bioepis’ European sales and marketing partner Biogen is swiftly working to introduce Flixabi across Europe, going in direct competition against Celltrion’s Remsima as well as J&J’s original drug. As of the second quarter of 2016, Celltrion’s Remsima had taken away some 40 percent of the original’s sales in Europe as the only Remicade biosimilar available in the region, the company said. And it claims it can drive this number up to 50 percent by the year’s end.
“As a ‘first mover’ product, Remsima has been accumulating prescription data over several years, boosting its credentials among both doctors and patients,” Celltrion said in a statement. “Given this, Remsima could take away some 50 percent of the original drug’s sales this year.”
However, the market entry of Samsung’s Flixabi could dim Remisma’s sales outlook in Europe, as it presents widened options to doctors treating patients diagnosed with conditions which require Remicade.
So far, Flixabi has been commercialized in Germany, one of the biggest markets for biologics within Europe, with plans for further launches across Europe, Biogen told The Korea Herald on Wednesday.
Moreover, analysts say Remsima’s performance is impressive, yet limited in scope. Celltrion’s replicated drug has had little impact on the sales of blockbusters drugs Enbrel and Humira, as was hoped, Samsung Securities analyst Kim Seung-woo pointed out in a February report.
Kim had forecast that Enbrel and Humira could be prescribed interchangeably with Remicade to treat autoimmune diseases like rheumatoid arthritis and ankylosing spondylitis, suggesting expanded market reach for Celltrion’s Remicade biosimilar, Kim said.
However, doctors in Europe appear to be cautious in prescribing Remsima in place of other anti-TNF biologic drugs (including Enbrel and Humira), given biosimilars are still new to the market, the analyst said.
Furthermore, biosimilar replications of the two drugs, produced by other global biopharma developers, have already entered, or are close to entering markets in Europe.
This year, the EMA approved two Enbrel biosimilars, each produced by Samsung Bioepis and Novartis-owned Sandoz. The former began selling its Enbrel replica -- Benepali -- in Europe from February, while the latter is making due preparations.
As for Humira, US pharmaceuticals giant Amgen and Samsung Bioepis are both awaiting the EMA’s permission to launch their Humira biosimilars.
“With the entry of Samsung’s Enbrel biosimilar this year and the debut of Humira biosimilars next year, Remsima’s sales will be further limited solely to the Remicade market,” Kim said.
Meanwhile in the US, Celltrion’s Remsima is poised to become the first Remicade biosimilar to be sold in the country, for the time being.
Having won a key patent dispute against Remicade’s original maker Janssen, a subsidiary of J&J, Celltrion and its sales partner Pfizer say they are on track in launching Remsima, to be sold under the name Inflectra, in the US by October.
The Korean drugmaker said it expects Inflectra to channel in some 2 trillion won ($1.79 billion) in annual profits in the US, taking away roughly 30 percent of Remicade’s US sales estimated at 5.4 trillion won.
Inflectra is expected to be sold at a price that is 15 to 20 percent cheaper than the original Remicade, HMC Investment Securities analyst Kang Yang-koo said in a June report.
Despite its early lead, Celltrion is now being chased by Samsung Bioepis, which applied for the US FDA’s regulatory approval of its Remicade biosimilar in March of this year.
Kang forecasts the Samsung unit will take around two years -- one year to gain regulatory approval and another year for commercialization preparations -- to officially debut its Remicade biosimilar in the US market, as was the case with Celltrion.
Race for additional biosimilars
Looking ahead, Celltrion is poised to become the first company to debut two additional biosimilars by as early as next year -- one referencing Roche’s lymphatic cancer treatment Rituxan and another based on Roche’s breast cancer drug Herceptin.
Despite its initial lead, the Korean drug maker may soon lose its “first mover” status as its two biosimilars again face quick-moving competition from the world’s biggest pharmaceuticals giants such as Novartis and Amgen.
Celltrion submitted its Rituxan biosimilar -- Truxima -- to the EMA in November 2015 and expects the European regulatory body to approve the drug by the year’s end. The firm plans to submit Truxima to the US FDA by the first quarter of 2017 as well.
Trailing behind by about six months in the segment is Novartis-owned Sandoz, which submitted its own Rituxan biosimilar to the EMA for approval in May of this year, and is expected to do the same for the US FDA in the near future.
Global pharmaceuticals giants Pfizer and Amgen are also wrapping up third-phase clinical trials of their own Rituxan biosimilars, with plans to commercialize their drugs in both Europe and the US in the coming future.
Meanwhile, no company has yet to seek regulatory approval of a Herceptin biosimilar in both Europe and the US, though Celltrion may become the first to do so.
The Korean biopharma developer said it plans to submit its Herceptin biosimilar -- Herzuma -- to the EMA before September. Celltrion began Herzuma’s third-phase clinical trials back in December 2011.
Set to quickly follow in the EU approvals process is Amgen, which began its third-phase clinical trials months later in July 2012. Pfizer and Samsung Bioepis are also preparing to launch their own Herceptin biosimilars, though their launches are expected at a slower pace.
By Sohn Ji-young/The Korea Herald (jys@heraldcorp.com)








![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)





![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)