Take a humane look at cosmetics
China reconsiders rules for mandatory animal testing
By Korea HeraldPublished : Jan. 7, 2014 - 19:24
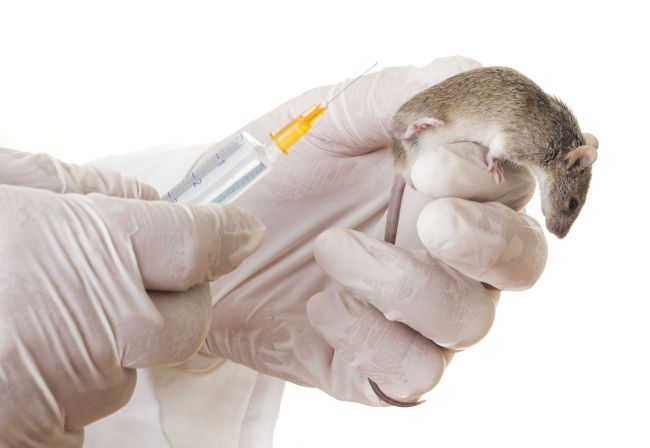
The China Food and Drug Administration issued a draft last month related to changes in the registration and licensing of cosmetics, following a ban on the sale of cosmetics developed through animal testing from the European Union in March.
The draft stated that cosmetics made from ingredients that have already been tested and classified as safe will be exempt from animal testing.
Even though the draft regulation, set to come into force in June, does not apply to cosmetics manufactured outside the Chinese mainland, or to “special-use” products such as hair dyes, sunscreens or skin-whitening products, it’s been hailed as a breakthrough by animal rights groups.
“The news from China marks a major milestone in our campaign and could constitute a significant watershed in our global efforts to end cosmetics animal testing worldwide,” said Troy Seidle, the Humane Society International’s Be Cruelty-Free director, in an online news statement.
The Body Shop, Lush, MooGoo and other companies, plus the United States-based nonprofit organization Humane Society International, have campaigned for decades for the mandatory testing of cosmetics on animals to be phased out worldwide.
Many cosmetic companies, such as The Body Shop and Lush, have turned down lucrative opportunities in the Chinese mainland on ethical grounds because in China all imported cosmetics products are subject to mandatory tests that use animals as the subjects. Wang Yiwen, a financial consultant at Deutsche Bank in Beijing, is a loyal customer of The Body Shop, but like all the brand’s fans in China, she either has to rely on friends traveling overseas or on agents at online marketplaces to make her purchases. “It’s a pity they haven’t officially entered the Chinese mainland market yet. Most people I know have to buy their products through online agents,” said Wang.
“We are delighted to hear that the Chinese government is looking at its policies regarding animal testing. Many animals could be saved from pain and death by these changes. For Lush, it brings the day nearer when we can, perhaps, trade in China,” wrote Hilary Jones, global ethical director for the U.K. cosmetics producer Lush Retail, in an emailed comment.
The Humane Society International estimates that around 300,000 animals are used every year in cosmetics testing in China. The country’s stance on animal testing remains the biggest hurdle to the promotion of alternative methods, according to experts.
“Currently, China has no law or regulation requiring alternative methods to be made mandatory, so there hasn’t been a huge uptake of those methods,” said Jiao Hong, director of the food laboratory at Guangdong Entry-Exit Inspection and Quarantine Bureau.
Strict safety tests
The Hygienic Standards for Cosmetics, China’s guidelines for safety tests on cosmetics, were introduced by the former Ministry of Health in 2007.
The methods specified by the regulation, which is based on guidelines drawn up by the Organization for Economic Cooperation and Development, require that all cosmetic products be subjected to 17 animal-based toxicological tests, such as those for acute oral toxicity, acute eye irritation, skin sensitization and a combined test for chronic toxicity and carcinogenicity.
The skin sensitization test uses 20 guinea pigs as samples and a further 10 as “controls” ― that is, they don’t undergo the tests. Cosmetics are repeatedly applied to a shaved area on the subject animal’s back, the condition of the skin is then compared with its control and any changes are noted.
In the combined chronic toxicity/carcinogenicity test, both the test and control groups comprise 100 animals, a 50-50 split of males and females. The tests are conducted continually throughout the animals’ life spans, usually about a year.
Li Hua, president of Animal Guardians, a nongovernmental animal rights group, said the problems associated with the protection of lab animals stem from the fact that the general public knows little about what happens in the labs.
“Even animal right activists such as my organization are unable to gain access to inside information, so the industry is effectively closed to outsiders,” she said.
Improvements in animal welfare were first introduced by the Ministry of Science and Technology, which issued China’s first guidelines on the humane treatment of lab animals ― including advice on breeding, transportation and the conduct of the experiments ― in October 2006.
Since then, the authorities in a number of areas, including the provinces of Guangdong and Hubei, and Beijing, have formulated their own regulations.
Compared with the laws and regulations in Western countries, though, some species have been omitted from the list covered by the test regulations, according to He Zhengming, a researcher with the National Institutes for Food and Drug Control.
In an article published in 2011, he noted that the national and local standards include the most-commonly used species, but fail to cover animals such as Mongolian gerbils and domestic cats.
Search for alternatives
The EU ban on animal testing has forced producers to accelerate research into alternative technologies. The move is unlikely to have an immediate impact on companies with production units in China, though, because products tested before the ban came into force will remain on the shelves in Europe.
L’Oreal, which recently expanded a factory in Hubei province into its largest production base in the Asia-Pacific region, has developed a Chinese EpiSkin model, a facsimile of human skin constructed from Asian keratinocytes, the dominant cells in the outer layer of the skin.
In a news release, the company said EpiSkin can provide solid technical support for the new EU regulations because it can be used as a replacement for human and animal tissue in some tests, especially those related to corrosion and irritation of the skin. In Europe, the product has already been certified for use.
In 2011, the CFDA embarked on a project to identify alternatives to animal testing.
The project, headed by He Zhengming, is examining the possibility of setting up a special body to research alternatives to toxicological tests on animals. The group also reports on the latest developments in ongoing research methods and the application of alternative research and testing, plus conditions in laboratories.
Both He and the CFDA declined invitations to be interviewed on the project’s latest findings.
Jiao, the expert from Guangdong Entry Exit Inspection and Quarantine Bureau, said that the alternative methods have drawbacks. “The methods are still immature in terms of testing new ingredients in cosmetics, especially the methods of testing for chronic diseases,” she said.
Cruelty-free cosmetics
Many cosmetics companies warmly greeted the proposal to phase out mandatory animal tests among Chinese producers.
“We know that many Chinese people have already tried Lush products and liked them, so we would love to be able to sell in China,” said Jones from Lush Retail.
The Body Shop also welcomes the signals from the Chinese authorities and looks forward to selling its products in China one day, company spokeswoman Louise Terry told CNN.
However, the companies insisted they would not make their products available in China until the requirement for mandatory animal testing is abandoned.
If it comes to pass, the move could also be instrumental in allowing Chinese cosmetics to be marketed in Europe. The regulatory requirement for animal-based tests have long been an obstacle to that ambition, according to Peter Li, Humane Society International’s China policy expert, who said the society has been in contact with the CFDA since June 2012, most recently in September.
“We tried to convey the following message: China can regard the adoption of the established non-animal testing methods used in the European Union as a way of reducing costs, reducing animal suffering, and addressing the loss of market access for Chinese cosmetics,” he said.
However, a total ban on animal-based tests proposed by some producers and animal rights groups has drawn criticism, even within the EU.
Cosmetics Europe, a trade association that represents the interests of the European cosmetics industry, said the ban is potentially harmful.
“By implementing the ban at this time, the European Union is jeopardizing the industry’s ability to innovate, particularly for SMEs (small and medium-sized enterprises),” said Bertil Heerink, Cosmetic’s Europe’s director general, in a statement at the time the ban came into force.
While Chinese researchers have long studied ways to improve the welfare of laboratory animals, most of the work has been aimed at providing better living conditions, thus reducing the animals’ stress levels and improving the accuracy of the results, according to experts.
“The animal welfare we are conducting is entirely different to that of animal rights groups who hold up banners to protest testing on animals,” said He in a 2011 interview.
“They (the protesters) are doing this from the angle of extreme animal rights and they show an utter disregard for scientific development. From our point of view, improving animal welfare is a service to scientific development, because animals that live in a filthy environment with poor nutritional conditions will not provide accurate test figures,” he said, adding that the test methods used in China must accord with the rest of the world if the results are to be universally acknowledged.
While the proposal to end animal testing has raised concerns among consumers about the safety of the alternative methods, Jiao noted that all the ingredients have already undergone safety tests and most of the products can only be distinguished from one another by the proportions in which the ingredients are used.
“Even after animal testing, cosmetics can’t be guaranteed 100 percent safe,” she said. “The results can only be assessed from the reactions of the majority of users and, of course, reactions may differ among individuals.”
By Xu Wei and Zhang Lei
(China Daily)
The draft stated that cosmetics made from ingredients that have already been tested and classified as safe will be exempt from animal testing.
Even though the draft regulation, set to come into force in June, does not apply to cosmetics manufactured outside the Chinese mainland, or to “special-use” products such as hair dyes, sunscreens or skin-whitening products, it’s been hailed as a breakthrough by animal rights groups.
“The news from China marks a major milestone in our campaign and could constitute a significant watershed in our global efforts to end cosmetics animal testing worldwide,” said Troy Seidle, the Humane Society International’s Be Cruelty-Free director, in an online news statement.
The Body Shop, Lush, MooGoo and other companies, plus the United States-based nonprofit organization Humane Society International, have campaigned for decades for the mandatory testing of cosmetics on animals to be phased out worldwide.
Many cosmetic companies, such as The Body Shop and Lush, have turned down lucrative opportunities in the Chinese mainland on ethical grounds because in China all imported cosmetics products are subject to mandatory tests that use animals as the subjects. Wang Yiwen, a financial consultant at Deutsche Bank in Beijing, is a loyal customer of The Body Shop, but like all the brand’s fans in China, she either has to rely on friends traveling overseas or on agents at online marketplaces to make her purchases. “It’s a pity they haven’t officially entered the Chinese mainland market yet. Most people I know have to buy their products through online agents,” said Wang.
“We are delighted to hear that the Chinese government is looking at its policies regarding animal testing. Many animals could be saved from pain and death by these changes. For Lush, it brings the day nearer when we can, perhaps, trade in China,” wrote Hilary Jones, global ethical director for the U.K. cosmetics producer Lush Retail, in an emailed comment.
The Humane Society International estimates that around 300,000 animals are used every year in cosmetics testing in China. The country’s stance on animal testing remains the biggest hurdle to the promotion of alternative methods, according to experts.
“Currently, China has no law or regulation requiring alternative methods to be made mandatory, so there hasn’t been a huge uptake of those methods,” said Jiao Hong, director of the food laboratory at Guangdong Entry-Exit Inspection and Quarantine Bureau.
Strict safety tests
The Hygienic Standards for Cosmetics, China’s guidelines for safety tests on cosmetics, were introduced by the former Ministry of Health in 2007.
The methods specified by the regulation, which is based on guidelines drawn up by the Organization for Economic Cooperation and Development, require that all cosmetic products be subjected to 17 animal-based toxicological tests, such as those for acute oral toxicity, acute eye irritation, skin sensitization and a combined test for chronic toxicity and carcinogenicity.
The skin sensitization test uses 20 guinea pigs as samples and a further 10 as “controls” ― that is, they don’t undergo the tests. Cosmetics are repeatedly applied to a shaved area on the subject animal’s back, the condition of the skin is then compared with its control and any changes are noted.
In the combined chronic toxicity/carcinogenicity test, both the test and control groups comprise 100 animals, a 50-50 split of males and females. The tests are conducted continually throughout the animals’ life spans, usually about a year.
Li Hua, president of Animal Guardians, a nongovernmental animal rights group, said the problems associated with the protection of lab animals stem from the fact that the general public knows little about what happens in the labs.
“Even animal right activists such as my organization are unable to gain access to inside information, so the industry is effectively closed to outsiders,” she said.
Improvements in animal welfare were first introduced by the Ministry of Science and Technology, which issued China’s first guidelines on the humane treatment of lab animals ― including advice on breeding, transportation and the conduct of the experiments ― in October 2006.
Since then, the authorities in a number of areas, including the provinces of Guangdong and Hubei, and Beijing, have formulated their own regulations.
Compared with the laws and regulations in Western countries, though, some species have been omitted from the list covered by the test regulations, according to He Zhengming, a researcher with the National Institutes for Food and Drug Control.
In an article published in 2011, he noted that the national and local standards include the most-commonly used species, but fail to cover animals such as Mongolian gerbils and domestic cats.
Search for alternatives
The EU ban on animal testing has forced producers to accelerate research into alternative technologies. The move is unlikely to have an immediate impact on companies with production units in China, though, because products tested before the ban came into force will remain on the shelves in Europe.
L’Oreal, which recently expanded a factory in Hubei province into its largest production base in the Asia-Pacific region, has developed a Chinese EpiSkin model, a facsimile of human skin constructed from Asian keratinocytes, the dominant cells in the outer layer of the skin.
In a news release, the company said EpiSkin can provide solid technical support for the new EU regulations because it can be used as a replacement for human and animal tissue in some tests, especially those related to corrosion and irritation of the skin. In Europe, the product has already been certified for use.
In 2011, the CFDA embarked on a project to identify alternatives to animal testing.
The project, headed by He Zhengming, is examining the possibility of setting up a special body to research alternatives to toxicological tests on animals. The group also reports on the latest developments in ongoing research methods and the application of alternative research and testing, plus conditions in laboratories.
Both He and the CFDA declined invitations to be interviewed on the project’s latest findings.
Jiao, the expert from Guangdong Entry Exit Inspection and Quarantine Bureau, said that the alternative methods have drawbacks. “The methods are still immature in terms of testing new ingredients in cosmetics, especially the methods of testing for chronic diseases,” she said.
Cruelty-free cosmetics
Many cosmetics companies warmly greeted the proposal to phase out mandatory animal tests among Chinese producers.
“We know that many Chinese people have already tried Lush products and liked them, so we would love to be able to sell in China,” said Jones from Lush Retail.
The Body Shop also welcomes the signals from the Chinese authorities and looks forward to selling its products in China one day, company spokeswoman Louise Terry told CNN.
However, the companies insisted they would not make their products available in China until the requirement for mandatory animal testing is abandoned.
If it comes to pass, the move could also be instrumental in allowing Chinese cosmetics to be marketed in Europe. The regulatory requirement for animal-based tests have long been an obstacle to that ambition, according to Peter Li, Humane Society International’s China policy expert, who said the society has been in contact with the CFDA since June 2012, most recently in September.
“We tried to convey the following message: China can regard the adoption of the established non-animal testing methods used in the European Union as a way of reducing costs, reducing animal suffering, and addressing the loss of market access for Chinese cosmetics,” he said.
However, a total ban on animal-based tests proposed by some producers and animal rights groups has drawn criticism, even within the EU.
Cosmetics Europe, a trade association that represents the interests of the European cosmetics industry, said the ban is potentially harmful.
“By implementing the ban at this time, the European Union is jeopardizing the industry’s ability to innovate, particularly for SMEs (small and medium-sized enterprises),” said Bertil Heerink, Cosmetic’s Europe’s director general, in a statement at the time the ban came into force.
While Chinese researchers have long studied ways to improve the welfare of laboratory animals, most of the work has been aimed at providing better living conditions, thus reducing the animals’ stress levels and improving the accuracy of the results, according to experts.
“The animal welfare we are conducting is entirely different to that of animal rights groups who hold up banners to protest testing on animals,” said He in a 2011 interview.
“They (the protesters) are doing this from the angle of extreme animal rights and they show an utter disregard for scientific development. From our point of view, improving animal welfare is a service to scientific development, because animals that live in a filthy environment with poor nutritional conditions will not provide accurate test figures,” he said, adding that the test methods used in China must accord with the rest of the world if the results are to be universally acknowledged.
While the proposal to end animal testing has raised concerns among consumers about the safety of the alternative methods, Jiao noted that all the ingredients have already undergone safety tests and most of the products can only be distinguished from one another by the proportions in which the ingredients are used.
“Even after animal testing, cosmetics can’t be guaranteed 100 percent safe,” she said. “The results can only be assessed from the reactions of the majority of users and, of course, reactions may differ among individuals.”
By Xu Wei and Zhang Lei
(China Daily)
-
Articles by Korea Herald







![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)






![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)